First enrolments to PrEPVacc clinical trial at Masaka
The first enrolments to PrEPVacc’s clinical trial have taken place at the Masaka site of the MRC/UVRI and LSHTM Uganda Research Unit.
Two participants were enrolled and vaccinated on Tuesday 15 December, the first in a hoped-for minimum of 1,668 participants across five planned study sites.
PrEPVacc is the first HIV efficacy trial ever to be conducted in East African countries. Besides Masaka, the PrEPVacc trial is planned to be conducted at Mbeya, Tanzania; Dar-es-Salaam, Tanzania; Maputo, Mozambique and Durban, South Africa.
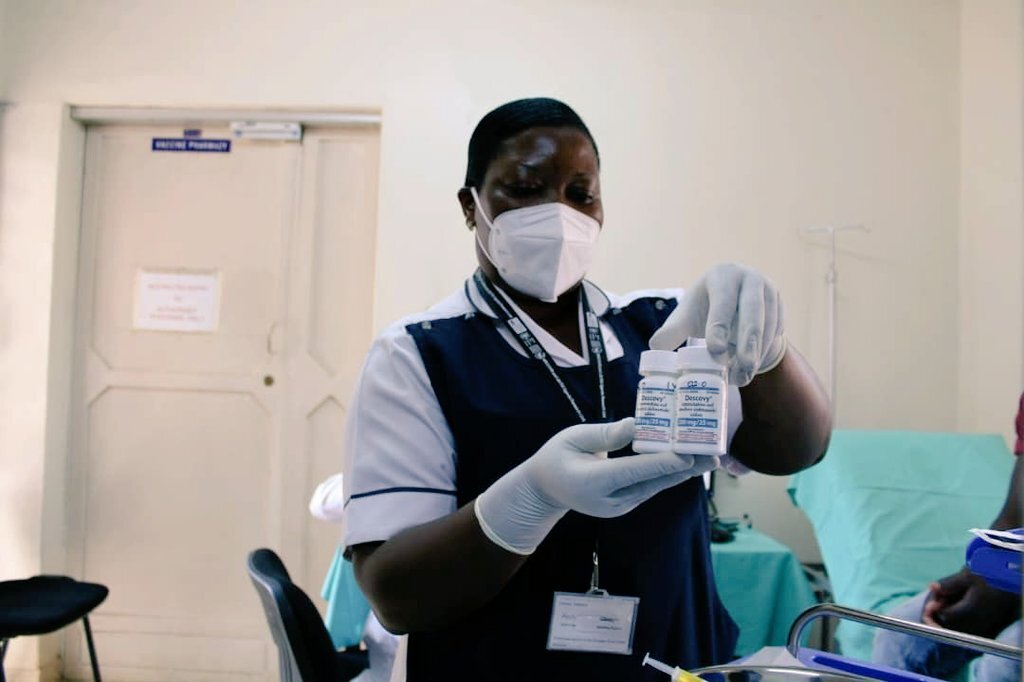
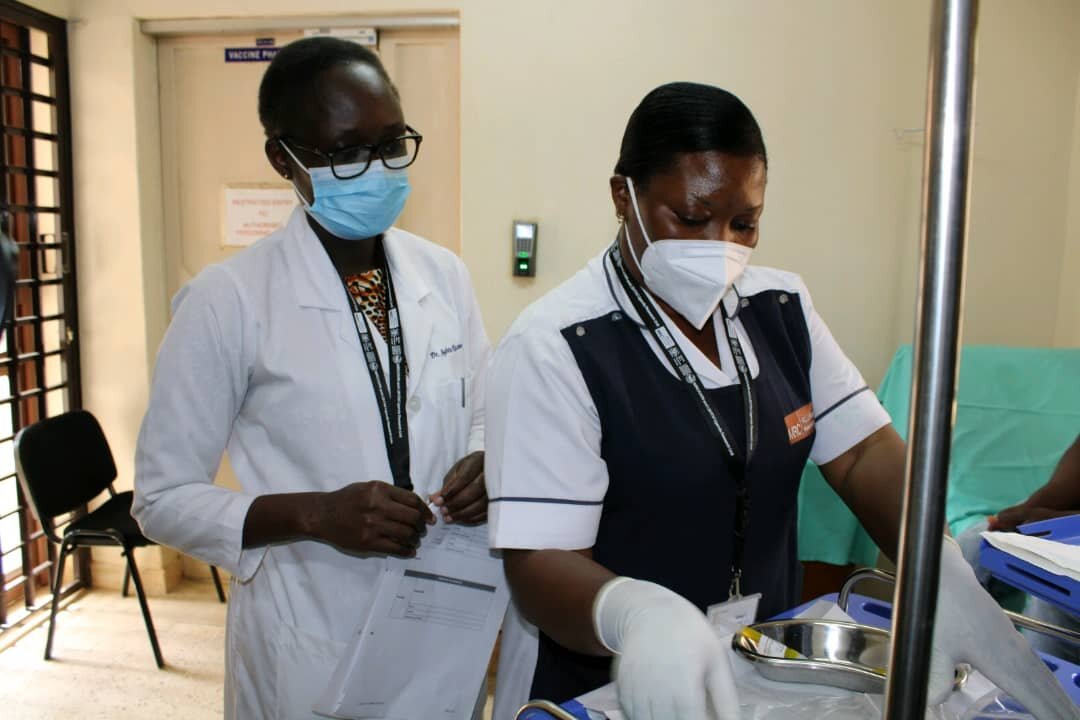
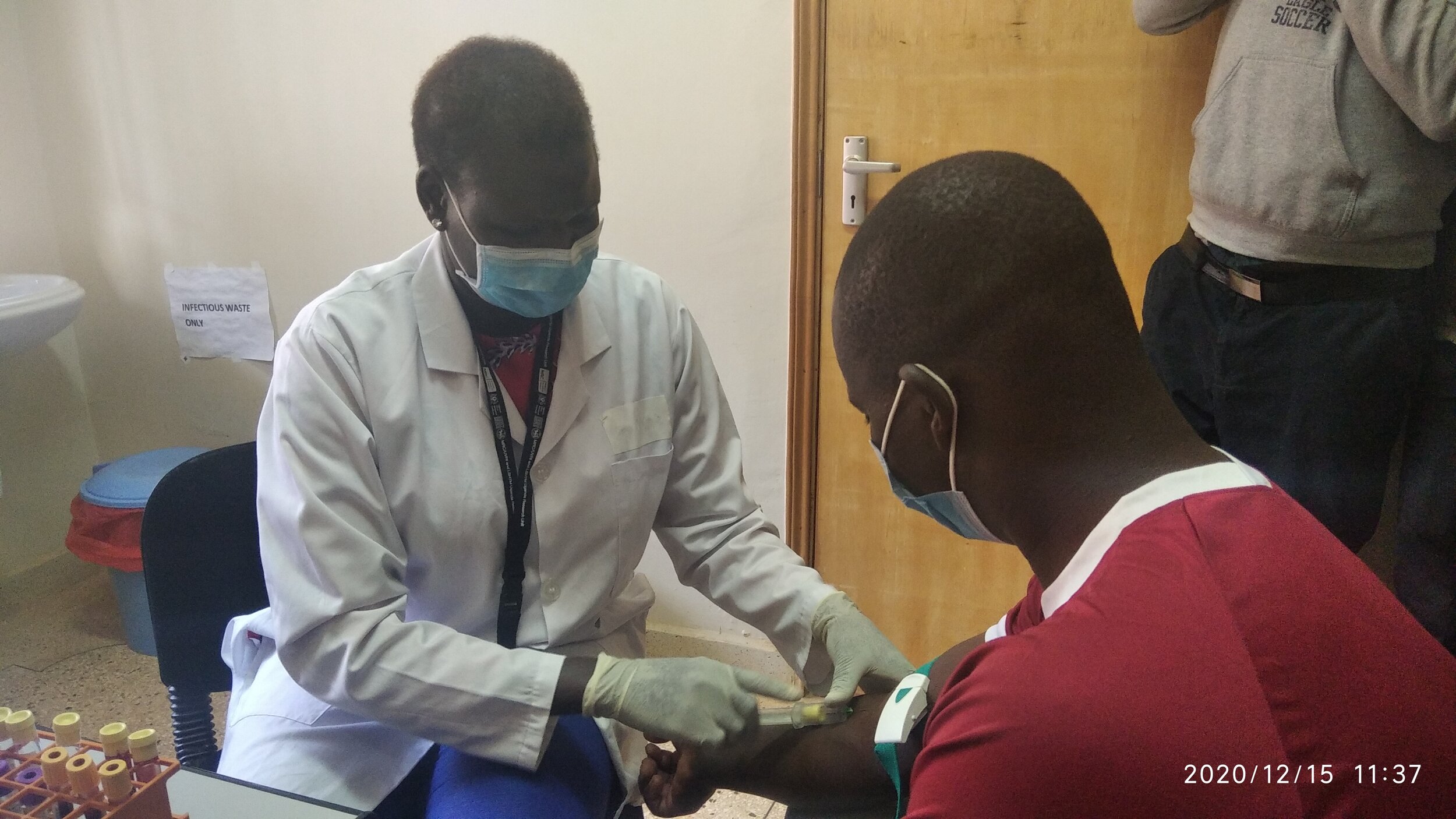
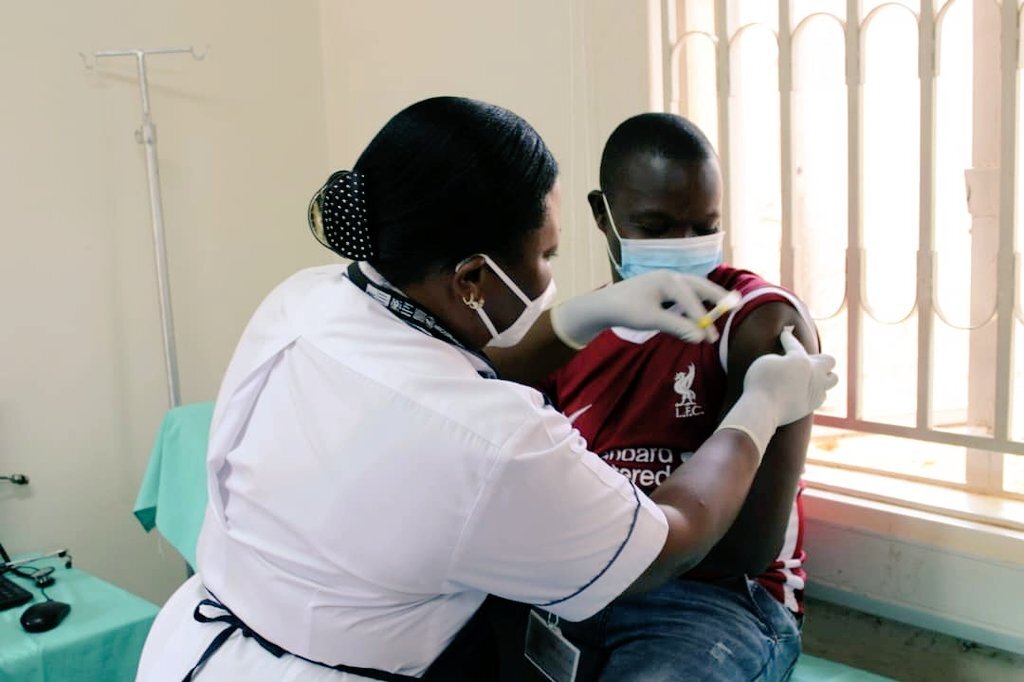
To mark the start of the clinical trial, PrEPVacc’s leaders at the site also briefed the media and gave a guided tour of the facilities in Masaka. In a news release issued by the site, Professor Pontiano Kaleebu, PrEPVacc Chief Investigator, said:
“PrEPVacc provides two great opportunities; first, for Africans to be able to participate and lead in the first HIV prevention trial to test two ways to prevent HIV, a scourge that has ravaged the continent and secondly, an opportunity to grow the capacity of African sites to do future trials themselves and to foster our own future leaders.”
- Prof Pontiano Kaleebu, PrEPVacc Chief Investigator


Link to MRC/UVRI news release: African-led trial to test two ways of preventing HIV at the same time, launches in Uganda (15 December 2020)
